|
Mysterious
and changing
TOURMALINE Roger
Warin
Introduction
Fig.
1 Elbaite San Piero, Elba Island, Tuscany, Italy. Coll. &
© R.
Warin. Blooming
at the end of the crystallization of pegmatites, tourmaline is multiple,
fascinating and mysterious. Its crystallochemistry is varied and complex, so the
amateur understands it with difficulty. Its prismatic, but often rounded
appearance, and its captivating and changing colors seduce as much the visitor
of the exhibitions or other shows than its composition disarms the systematic
collector. It remains difficult to name it precisely to the point that it
requires a crystallochemical analysis to clarify its identity. Even its chemical
formula is a puzzle. It takes almost a full line to write it. To
remember its composition by heart asks for more than a single mnemonic trick or,
alternatively, this requires a careful deep thought! To tell the truth, there
are more than two dozen tourmalines, gathered in the Tourmaline Supergroup. But
ancient usage has defined poorly defined varieties, based mainly on the hues of
the crystal. Since gemstone tourmaline is a fine stone, gemologists often use
this vernacular nomenclature, conveyed by usage. The rubellite is red and the
indicolite is blue while the verdelite is green (often dark).
Fig.
2 Elbaite, herderite - Pederneira Mine, Sao Josι da Safira, Minas Gerais,
Brazil, The
Tourmaline Supergroup This
group is so vast that it occupies almost two pages of Fleisher's Glossary
(2014). The reader will refer to this lexicon to find its chemical formulas.
Tourmalines belong to the family of cyclosilicates, whose characterization is
the cyclic sequence of several [SiO4]
groups. In this case, the cyclic silicate anion of tourmalines connects 6 [SiO4]
tetrahedra. This polymerization leads to an anion of formula (Si6O18)12- centered on the vertical ternary axis c. These anionic planes are perpendicular to the c axis and the upper face is indexed (0001). Another essential element for the "tourmalinization" of silicates (for example, quartz, feldspar and micas in granites) is the presence of boron by a metasomatism process. Boron is supplied by the hydrothermal fluids in the ends of crystallizations of acid magmas producing granites. The presence of boron is often possible because it is a lithophilic element. Tourmaline is therefore also built on planes made of plane (BO3)3- anions alternating with the coplanar cyclosilicates rings (Si6O18)12-. The structure accepts 3 triangular anions (BO3)3- per ring (Si6O18)12-.
All
these negative charges are obviously compensated by positive charges introduced
by various cations. And it is here that the whole variety of substitution
appears, giving free course to the very great fantasy of chance at the end of
the crystallizations of pegmatites. Tourmaline is considered as the garbage can
of pegmatites, accepting chemical elements refused by other crystalline
structures. This
anionic lasagna is neutralized by 3 types of cations: X, Y and Z, with: X
= Na+ and
more rarely, Ca2+. Y
= Li+,
Mg2+,
Fe2+,
Fe 3+,
Mn3+,
Cr3+,
Al3+,
etc. Z
= Al3+,
Fe3+,
Cr3+,
V3+,
etc. Since
the electrical neutrality is still not completely obtained, the addition of
additional small anions such as OH-,
F-,
O2- is
necessary. We therefore arrive at the classic general formula: X
Y3 Z6 [(BO3)3 (Si6O18)] (OH,
F, O)4 And
for example, elbaite has the formula: Na
(Al,Li)3 Al6 [(BO3)3 (Si6O18)]
(OH)4 Cations
size is also decisive for their insertion into the cavities left between the
various sheets of the anionic structure. Geometric illustrations will enhance
the reader representation of the situation. When the general structure of
tourmaline is understood, it becomes easier to see the insertion volumes for
left for the cations. Anecdotally, when I was young, the extended formula of
tourmaline was sometimes required by a professor wishing to put an end to the
examination... To better perceive below the main characteristics of such a
dense, while very ordered, cluster of varied atoms, the complexity of the
following drawings will increase gradually. Cristallochemistry
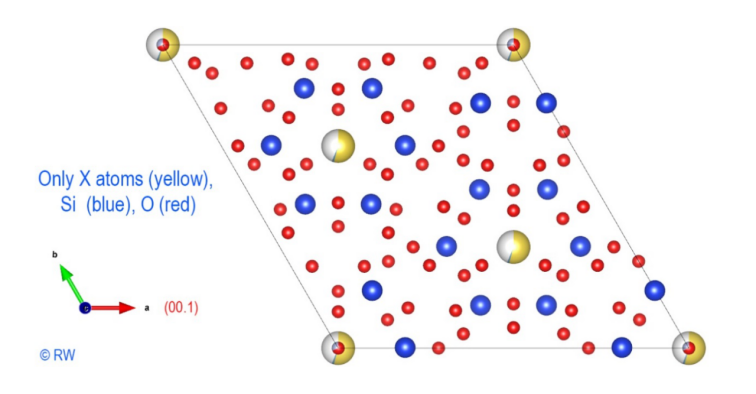
Fig.
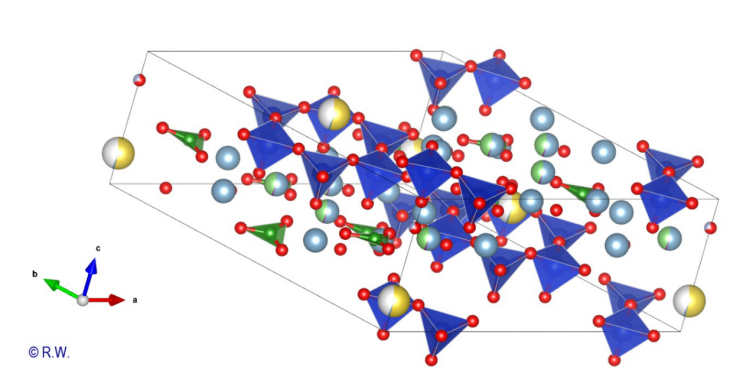
3 Elbaite Perpendicular section to the vertical "c" axis A
first structural sketch of tourmaline, expressed in the case of elbaite, is
shown in the figure (Fig. 3). To simplify the scheme, boron and aluminum atoms
have been omitted. Only the X atoms (yellow), Si (blue) and O (red) remain. Thus
the hexagonal ring of the anion (Si6O18)12- appears
clearly, highlighting the cyclic nature of the cyclosilicate anion. The vertical
ternary c axis is centered perpendicular to this cycle. This figure is a
projection and all the atoms are not in the same plane. An upcoming figure will
show the expanded structure after a rotation of the drawing. Now,
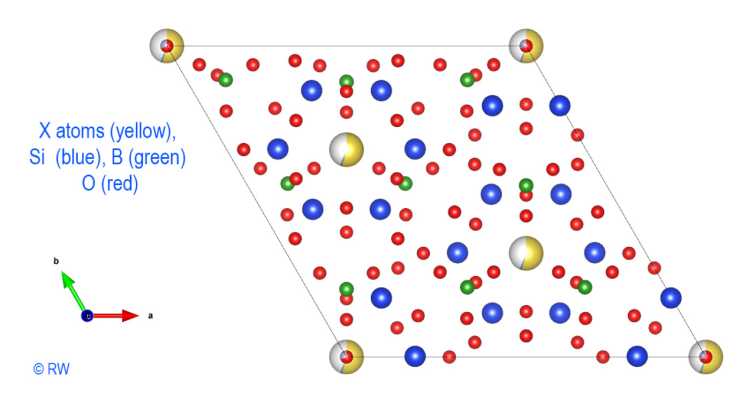
let's add boron atoms (Fig. 4). They form the borate anions (BO3)3- planes.
They are placed in the center of three O atoms and complete the anionic layer.
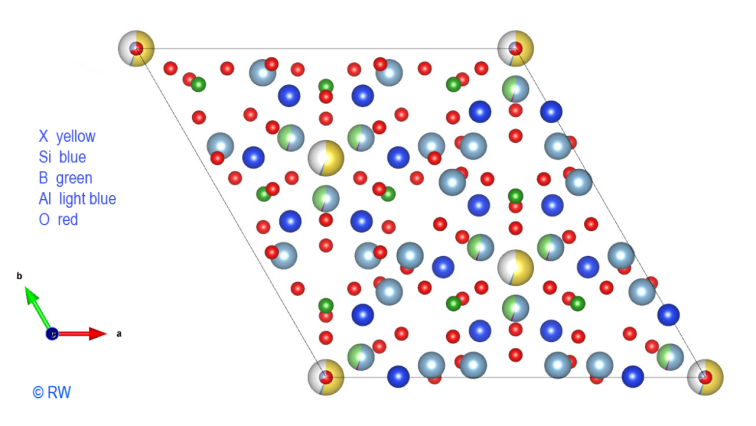
The addition of Al3+ cations
is shown in the following drawing (Fig. 5). Readability is getting lower and the
discernment of atomic associations becomes more difficult. It should be noted
that the symmetry of the structure is preserved in its slightest details. It
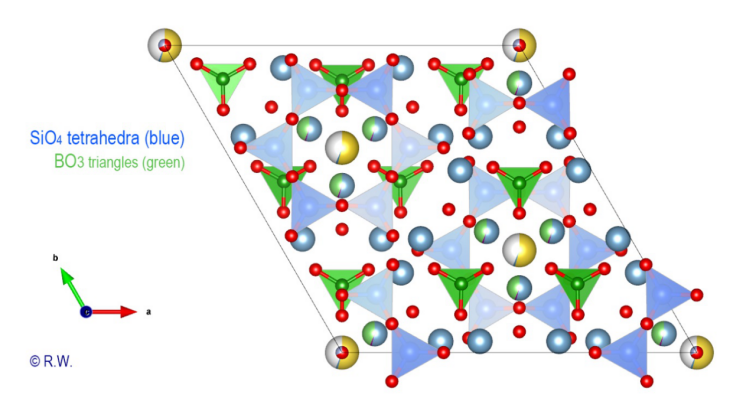
remains possible to emphasize the cyclosilicate character by selectively drawing
the silicon polyhedra (Fig. 6). The
spatial structure of tourmaline is useful in more than one respect. It confirms
the absence of a plan of weakness that would favor a cleavage, since we observe
that its fracture is conchoidal. But there is another property that is
remarkably highlighted: tourmaline has a low symmetry since it belongs to the
rhombohedral R3m class.
Unit cell parameters vary by species between: a
= 15.81 16.10 Ε c = 7.09 7.25 Ε Z = 3 hardness
= 7.5 very bad cleavages on {11.0} et {10.1}. Very
rare twins on {10.1} and {40.1}.
Fig.
4 Elbaite Perpendicular section to « c » axis without Al
atoms. © R.W.
Fig.
5 Elbaite Perpendicular
section to the vertical "c" axis.
Fig.
6 Elbaite Crystal structure with the SiO4 tetrahedra
of Elbaite
is highly piezoelectric and pyroelectric. These last properties are typical of
minerals that do not have a symmetry Center, which means that no face has any
other parallel face. The pinacoid (crystallographic form with 2 parallel faces)
does not exist in this case and it is replaced by the pedion (crystalline
form characterized by a single face). So, the two basal ends of the prism are
different to the point of bearing different names, the analogous
pole (the
upper pole - whose electric potential is of the same sign as the temperature
change) and the antilogous
pole (which
potential varies as the opposite of the temperature change). This behavior is
called pyroelectricity.
In the figure (Fig. 6) we can see the triangular bases of [SiO4]
tetrahedra are all oriented in the same direction. An angled view shows the
profile of the unit cell (Fig. 7). Piezoelectricity is
the phenomenon of appearance of electrical charges at the poles of a tourmaline
crystal when it is subjected to pressure and vice versa. The application of an
electrical voltage at the ends of the crystal induces a change in its dimensions
(a feature widely used for quartz that also does not have a center of symmetry).
The tourmaline of Madagascar was used for the manufacture of the sonars used
during the First World War. In
profile view, the cell reveals its secrets. Above and at the center of the
cyclosilicate ring, a more bulky cation is seen, that is sodium the Na+
ion
in the case of elbaite. The three triangular (BO3)3- anions
which are better seen in the previous figure (Fig. 6), are located at the lower
end of the [SiO4]
tetrahedra. Na atoms are located at the X sites. The X spheres of the general
sketch (Fig. 7) are two-colored, because the X sites can be
occupied by Na or Ca or can be left vacant, depending on the tourmaline species.
Fig.
7 Pivoting the unit cell of elbaite. © R.W.
Fig.
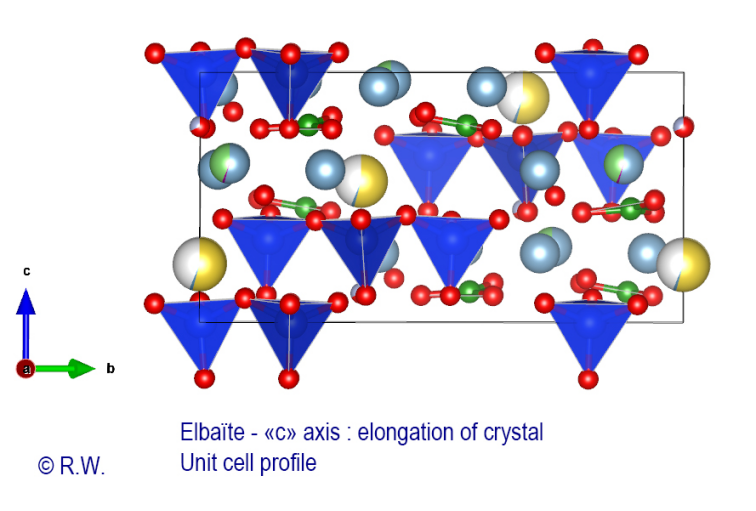
8 Unit cell profile of elbaite with in blue the tetrahedra [SiO4], The
following figure (Fig. 8) is much more explicit because it shows in profile the
contents of this unit cell. The [SiO4]
(blue) tetrahedra, all
oriented in
the same direction, namely the tip directed towards the (lower) antilogue pole,
are easily noticeable. It is this feature of the structure that prohibits the
presence of a Symmetry Center. We also see that the borate anions are not
rigorously coplanar with cyclosilicates rings, because of the deformations
induced by bulky X cations. The oxygen atoms are stratified and they participate
in the coordination bonds of all the atoms of this complex building. The Al3+ cations
occupy the other spaces of this trigonal structure (Fig. 8). Coordinating
polyhedra Na-O9 Polyhedron:
Each anionic group [(BO3)3 (Si6O18)]
is attached to an X cation (in yellow, Fig. 8) located slightly above it. It is
the bulky Na+ cation
which is located on this X-O9
site,
a coordination polyhedron with 9 ligands. In short, it is centered above the
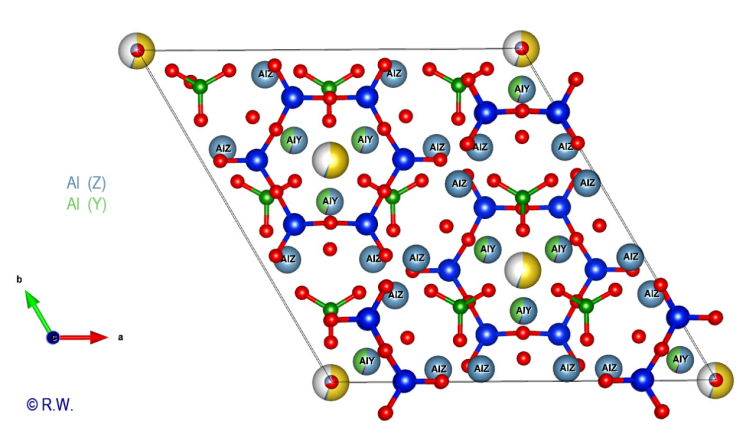
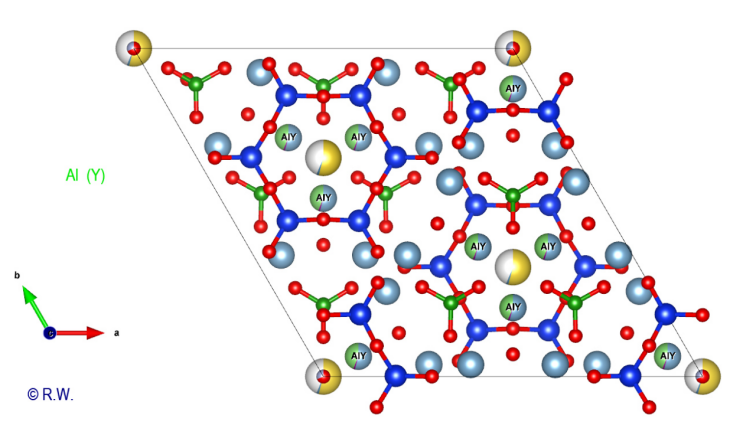
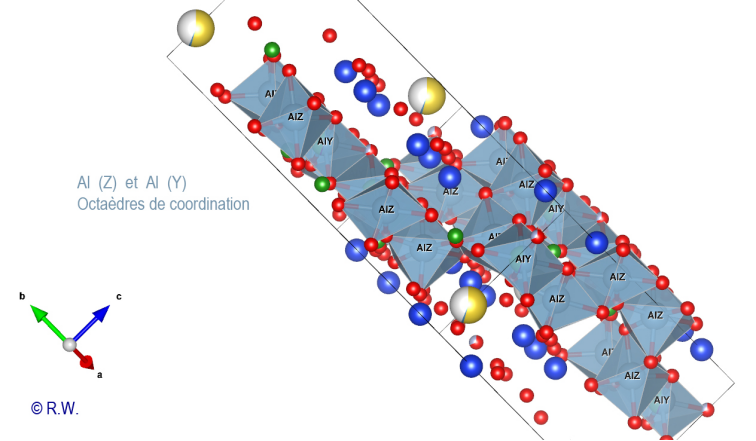
cyclosilicate anion. Al-O6 octahedra: The 9 elbaite octahedral sites Y and Z are occupied by aluminum atoms (Fig. 9). Like a double pyramid, an octahedron is a site of coordination with 6 bonds. A quick look at the general formula shows that two possible situations exist for Al3+, either the Z or Y sites. The figure (Fig. 10) highlights the location of the 3 Y sites, while the other (Fig. 11) shows the 6 Z sites.
Fig.
9 Elbaite - Polyhedral yellow sites X = Na+
Fig.
10 Elbaite Octahedral sites Y (slightly blue, annoted Y) : Al3+
©
R.W. These
considerations correspond to an ideal case (elbaite), because many cationic
substitutions (and even vacant sites symbolized by the sign
Fig.
11 Elbaite Al3+ in
octahedral sites Z. © R.W. These
observations demonstrate that in each of its details, trigonal symmetry is
obeyed. One may better understand that the main habitus of a crystal, as well as
the appearance of facets modifying it, are closely correlated to the intimate
structure of the building. The hexagonal cyclosilicate anion is surmounted by
the bulky cation Na+.
It is surrounded by 3 Al3+ in
Y sites whereas 6 Al3+ (Z)
encircle the cyclosilicate anion (Fig. 11). The
introduction of coordination polyhedra does not simplify the drawing (Fig. 12).
But seen in profile, this purged drawing of [SiO4]
tetrahedra illustrates the presence of the Al3+ cations
packaged in the spaces left by the anions. The whole structure of elbaite is
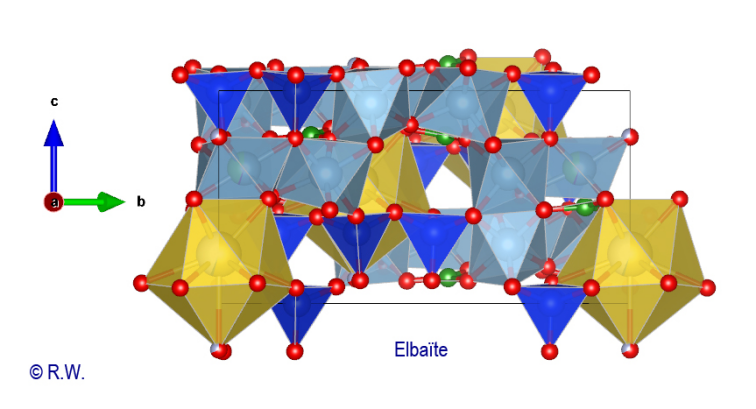
proposed in the figure (Fig. 13), with the Na polyhedra well displayed.
CONCLUSION The
classification of the various tourmalines is a tedious task that the reader will
approach on a case by case basis. The main constant is the anionic component.
These are borosilicates. After
much hesitation, the
mystery surrounding tourmaline prompted us to develop and present this
structural aspect, often touched but never presented, in the bulletin of the
Belgian club AGAB-minibul (2017, vol.50, 4, p.85-97 ). The main difficulty lies
in the reading of the spatial arrangement of the various atoms and groups of
atoms in the crystallographic drawings.
Fig.
13 Elbaite, profile view structure, with polyhedra [NaO9]
© R.W.
Fig.
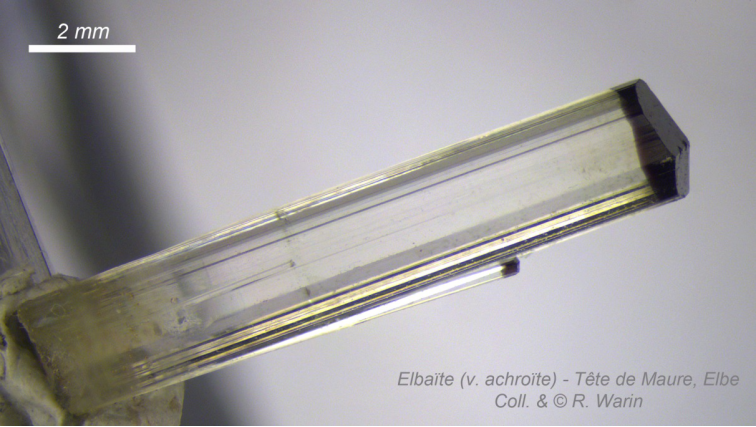
14 Elbaite (v. achroite) Moors head », Elba (It.) Coll.
& © R.W. Elbaite of the type locality (Elba Island, Tuscany, Italy) has its origin in sodolithium pegmatites which makes it much rarer than black schorlites (Y = Fe2+). In the Island of Elba it can take soft pastel colors or even be colorless (achroite variety) with often a black top, called "Moor's head" (Fig 14).
Fig.
15 Elbaite Cruzeiro Mine, Sao Jose da Safira, Doce Valley, Minas Gerais,
Brazil (10 cm). Postface
Fig.
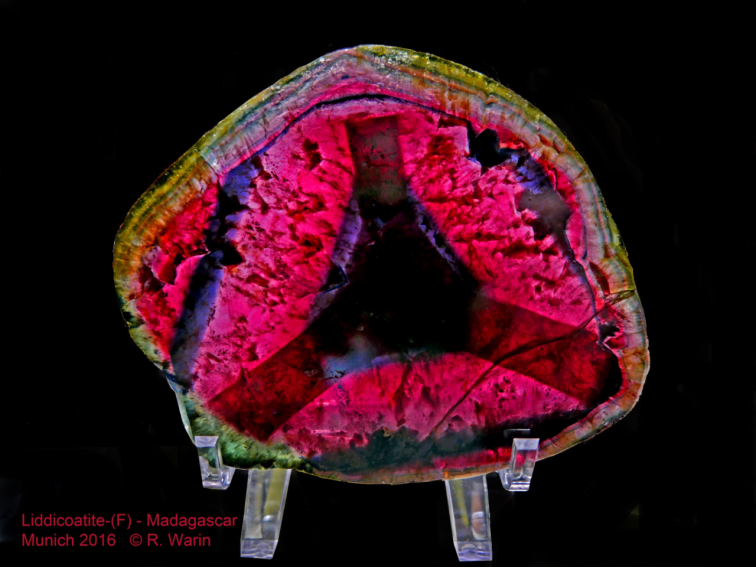
16 Liddicoatite-(F) (Madagascar), one of the many species of tourmaline. Munich
2016 © R. Warin. Reading
such an article is difficult and it will often be superficial. It assumes
"prerequisites" notions, but it appeared to me that certain facts
could be underlined to show the origin of the losses of symmetry of the crystal
and the appearance of mysterious and yet useful properties such as
pyroelectricity and piezoelectricity. In another well-known example of applied
piezoelectricity, quartz watches use the resonance of a quartz tuning fork to
create regular pulses. This resonator plays the role of the old balance wheels.
This property is at the base of the synthesis of many artificial compounds such
as ceramics or piezoelectric polymers. The industrial development of these
techniques is limitless. With quartz, tourmaline has allowed the discovery of
such phenomena. The first demonstration of the direct quartz piezoelectric
effect is due to Pierre and Jacques Curie in 1880.
|